Select the Name of the Family to Which the Following Compound Belongs
Classification of Arenes
- Folio ID
- 930
Benzene, CsixH6, is an organic aromatic compound with many interesting properties. Unlike aliphatic (straight concatenation carbons) or other cyclic organic compounds, the construction of benzene (3 conjugated π bonds) allows benzene and its derived products to be useful in fields such equally health, laboratory, and other applications such as rubber synthesis.
Introduction
Benzene derived products are well known to be pleasantly fragrant. For this reason, organic compounds containing benzene rings were classified as existence "effluvious" (sweetness smelling) amongst scientists in the early 19th century when a relation was established between benzene derived compounds and sweet/spicy fragrances. At that place is a misconception amongst the scientific community, still, that all aromatics are sweet smelling and that all sweet smelling compounds would have a benzene ring in its structure. This is simulated, since non-aromatic compounds, such as camphor, extracted from the camphor laurel tree, release a potent, minty aroma, however it lacks the benzene band in its construction (Figure one). On the other hand, benzene itself gives off a rather strong and unpleasant smell that would otherwise invalidate the definition of an aromatic (sugariness-smelling) compound. Despite this inconsistency, however, the term aromatic continues to be used today in guild to designate molecules with benzene-like rings in their structures. For a modernistic, chemical definition of aromaticity, refer to sections Aromaticity and Hückel's Rule.
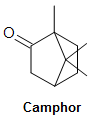
Figure 1. Top-view of camphor, along with its monoterpene unit. Notice how camphor lacks the benzene ring to exist "effluvious".
Many aromatic compounds are however, sweet/pleasant smelling. Eugenol, for instance, is extracted from essential oils of cloves and it releases a spicy, clove-like aroma used in perfumes. In addition, it is also used in dentistry equally an analgesic.
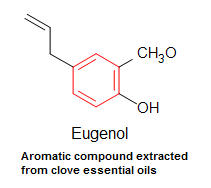
Figure two. Eugenol, an aromatic compound extracted from clove essential oils. Used in perfumes and as an analgesic. The benzene band is labeled in red in the eugenol molecule.
Is it cyclohexane or is information technology benzene?
Due to the similarity between benzene and cyclohexane, the two is often confused with each other in beginning organic chemistry students.

Figure 3. Structure comparison between cyclohexane and benzene
If you were to count the number of carbons and hydrogens in cyclohexane, you volition notice that its molecular formula is C6H12 . Since the carbons in the cyclohexane ring is fully saturated with hydrogens (carbon is leap to two hydrogens and ii adjacent carbons), no double bonds are formed in the cyclic ring. In dissimilarity, benzene is only saturated with i hydrogen per carbon, leading to its molecular formula of CviHhalf dozen . In order to stabilize this structure, 3 conjugated π (double) bonds are formed in the benzene band in order for carbon to have four adjacent bonds.
In other words, cyclohexane is not the aforementioned as benzene! These two compounds have different molecular formulas and their chemical and physical properties are not the same. The hydrogenation technique tin be used by chemists to catechumen from benzene to cyclohexane past saturating the benzene ring with missing hydrogens.
A special goad is required to hydrogenate benzene rings due to its unusual stability and configuration. Normal catalytic hydrogenation techniques will non hydrogenate benzene and yield any meaningful products.
What most Resonance?
Benzene tin be drawn a number of different ways. This is because benzene'southward conjugated pi electrons freely resonate within the cyclic band, thus resulting in its two resonance forms.
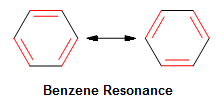
Figure 4. The Effigy to the left shows the two resonance forms of benzene. The delocalized electrons are moved from one carbon to the side by side, thus providing stabilization energy. Ring structures stabilized by the movement of delocalized electrons are sometimes referred to as arenes.
.png?revision=1&size=bestfit&width=70&height=70)
As the electrons in the benzene ring can resonate inside the ring at a adequately high charge per unit, a simplified annotation is oft used to designate the two different resonance forms. This notation is shown higher up, with the initial three pi bonds (#one, #2) replaced with an inner ring circle (#3). Alternatively, the circumvolve inside the benzene ring can as well exist dashed to testify the same resonance forms (#iv).
The Formation of the Phenyl Group and its Derivatives
The phenyl grouping can be formed past taking benzene, and removing a hydrogen from it. The resulting molecular formula for the fragment is C6H5. Annotation: Although the molecular formula of the phenyl group is C6Hfive, the phenyl group would e'er take something attached to where the hydrogen was removed. Thus, the formula is often written every bit Ph-R, where Ph refers to the Phenyl group, and R refers to the R group fastened to where the hydrogen was removed.
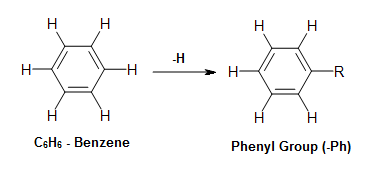
Figure 5. Effigy demonstrating the removal of hydrogen to class the phenyl group.
Different R groups on the phenyl group allows different benzene derivatives to be formed. Phenol, Ph-OH, or C6H5OH, for example, is formed when an booze (-OH) grouping displaces a hydrogen atom on the benzene ring. Benzene, for this very aforementioned reason, tin can be formed from the phenyl group by reattaching the hydrogen dorsum its place of removal. Thus benzene, similar to phenol, can exist abbreviated Ph-H, or C6H6.
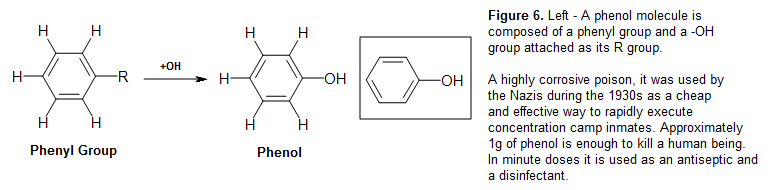
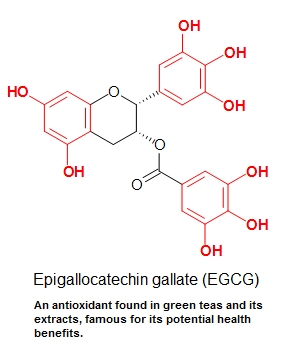
Figure 7: Epigallocatechin gallate (EGCG), an antioxidant found in dark-green teas and its extracts, is famous for its potential health benefits. The molecule is a type of catechin, which is equanimous of multiple phenol (labeled in cherry) units (polyphenols - see polycyclic aromatics). Since catechins are commonly found in plant extracts, they are ofttimes referred as plant polyphenolic antioxidants.
As you lot tin meet in a higher place, these are just some of the many possibilities of the benzene derived products that take special uses in man health and other industrial fields.
Classification of Benzene Derived Compounds
Unlike aliphatic organics, classification of benzene-derived compounds can be disruptive because a unmarried aromatic compound can have multiple possible names (such as mutual and systematic names) exist associated with its structure. In these sections, nosotros will analyze some of the means these compounds tin be named.
Simple Benzene Naming
Some common substituents, like NOii, Br, and Cl, tin can be named this way when information technology is attached to a phenyl group. Long chain carbons fastened can likewise exist named this way. The general format for this kind of naming is:
(positions of substituents (if >1)- + # (di, tri, ...) + substituent)n + benzene.
For example, chlorine (Cl) attached to a phenyl group would be named chlorobenzene (chloro + benzene). Since at that place is just one substituent on the benzene ring, we do not accept to indicate its position on the benzene ring (as it can freely rotate around and you would end upward getting the aforementioned chemical compound.)
.png?revision=1&size=bestfit&width=320&height=160)
.png?revision=1&size=bestfit&width=320&height=160)
Figure 8. Case of simple benzene naming with chlorine and NO2 as substituents.
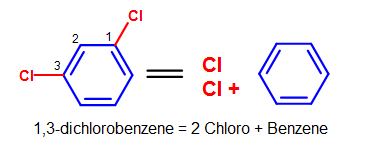
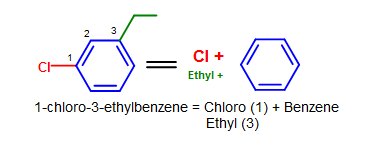
Figure 9. More complicated elementary benzene naming examples - Notation that standard nomenclature priority rules are applied here, causing the numbering of carbons to switch. Encounter Nomenclature of Organic Compounds for a review on naming and priority rules.
Ortho-, Meta-, Para- (OMP) Nomenclature for Disubstituted Benzenes
Instead of using numbers to bespeak substituents on a benzene ring, ortho- (o-), meta- (k-), or para (p-) can be used in place of positional markers when there are two substituents on the benzene ring (disubstituted benzenes). They are defined as the following:
- ortho- (o-): 1,2- (adjacent to each other in a benzene ring)
- meta- (m): ane,3- (separated by one carbon in a benzene ring)
- para- (p): 1,iv- (across from each other in a benzene ring)
Using the same example above in Figure 9a (i,3-dichlorobenzene), we tin employ the ortho-, meta-, para- nomenclature to transform the chemical proper noun into yard-dichlorobenzene, as shown in the Figure below.
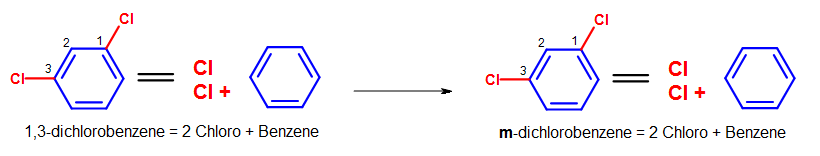
Figure 10. Transformation of i,3-dichlorobenzene into m-dichlorobenzene.
Here are some other examples of ortho-, meta-, para- nomenclature used in context:

Yet, the substituents used in ortho-, meta-, para- nomenclature do not take to exist the same. For example, we tin can utilise chlorine and a nitro group as substituents in the benzene ring.
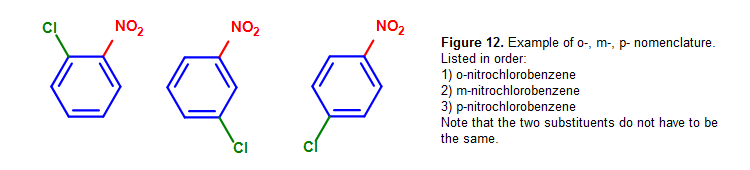
In decision, these can be pieced together into a summary diagram, as shown below:
.png?revision=1&size=bestfit&width=511&height=192)
Base Proper name Classification
In addition to elementary benzene naming and OMP nomenclature, benzene derived compounds are likewise sometimes used every bit bases. The concept of a base is like to the classification of aliphatic and cyclic compounds, where the parent for the organic compound is used as a base (a name for its chemic name. For instance, the post-obit compounds take the base names hexane and cyclohexane , respectively. See Nomenclature of Organic Compounds for a review on naming organic compounds.
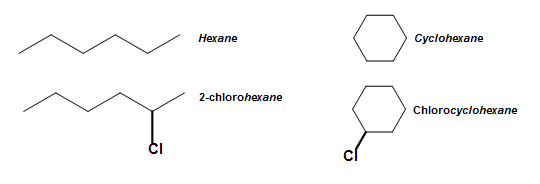
Benzene, like to these compounds shown above, likewise has base names from its derived compounds. Phenol (C6H5OH), as introduced previously in this commodity, for example, serves as a base when other substituents are attached to information technology. This is all-time illustrated in the diagram beneath.
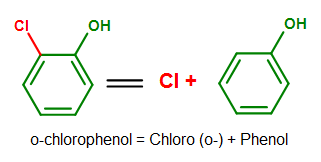
Figure fourteen. An example showing phenol as a base in its chemical name. Note how benzene no longer serves as a base when an OH grouping is added to the benzene ring.
Alternatively, we tin use the numbering system to bespeak this compound. When the numbering system is used, the carbon where the substituent is attached on the base will be given the first priority and named equally carbon #1 (C1). The normal priority rules so employ in the nomenclature process (give the rest of the substituents the lowest numbering equally you could).
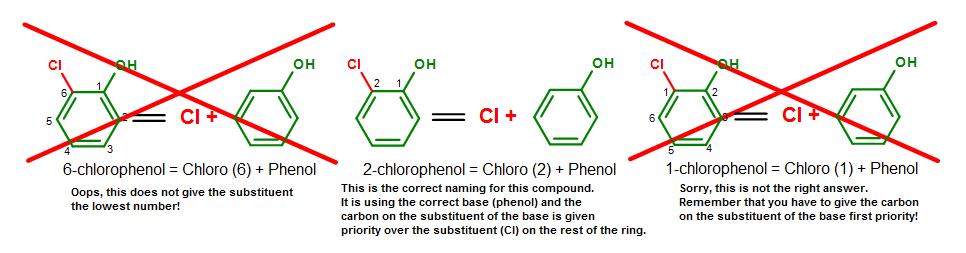
Figure 15. The naming process for 2-chlorophenol (o-chlorophenol). Annotation that 2-chlorophenol = o-chlorophenol.
Below is a list of usually seen benzene-derived compounds. Some of these mono-substituted compounds (labeled in red and green), such every bit phenol or toluene, can be used in identify of benzene for the chemic's base of operations name.
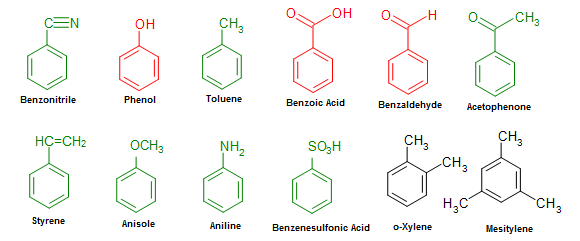
Figure 16. Common benzene derived compounds with various substituents.

Common vs. Systematic (IUPAC) Nomenclature
Co-ordinate to the indexing preferences of the Chemical Abstracts, phenol, benzaldehyde, and benzoic acid (labeled in red in Effigy 16) are some of the mutual names that are retained in the IUPAC (systematic) nomenclature. Other names such as toluene, styrene, naphthalene, or phenanthrene can also be seen in the IUPAC arrangement in the same way. While the utilize of other mutual names are usually acceptable in IUPAC, their use are discouraged in the nomenclature of compounds.
Nomenclature for compounds which has such discouraged names will be named by the simple benzene naming system. An instance of this would include toluene derivatives like TNT. (Note that toluene past itself is retained by the IUPAC nomenclature, just its derivatives, which contains additional substituents on the benzene ring, might be excluded from the convention). For this reason, the mutual chemical name 2,4,6-trinitrotoluene, or TNT, equally shown in Figure 17, would not be appropriate under the IUPAC (systematic) nomenclature.
In society to correctly name TNT nether the IUPAC system, the elementary benzene naming organization should be used:
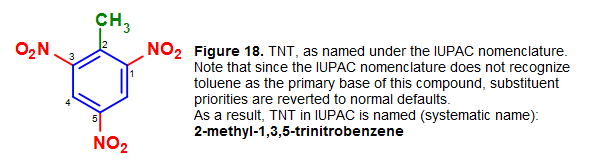
Effigy 18. Systematic (IUPAC) name of 2,four,half-dozen-trinitrotoluene (common name), or TNT. Note that the methyl group is individually named due to the exclusion of toluene from the IUPAC nomenclature.

Effigy nineteen. The common name 2,four-dibromophenol, is shared by the IUPAC systematic nomenclature. Only substituents phenol, benzoic acid, and benzaldehyde share this commonality.
Since the IUPAC classification primarily rely on the simple benzene naming organisation for the nomenclature of unlike benzene derived compounds, the OMP (ortho-, meta-, para-) system is non accepted in the IUPAC nomenclature. For this reason, the OMP system will yield common names that tin can be converted to systematic names past using the same method as in a higher place. For example, o-Xylene from the OMP system can be named 1,2-dimethylbenzene past using unproblematic benzene naming (IUPAC standard).
The Phenyl and Benzyl Groups
The Phenyl Group
Equally mentioned previously, the phenyl group (Ph-R, CsixH5-R) tin can be formed by removing a hydrogen from benzene and attaching a substituent to where the hydrogen was removed. To this phenomenon, we tin can name compounds formed this way past applying this rule: (phenyl + substituent). For example, a chlorine attached in this manner would exist named phenyl chloride, and a bromine fastened in this manner would be named phenyl bromide. (See below diagram)
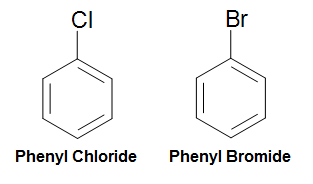
Effigy 20. Naming of Phenyl Chloride and Phenyl Bromide
While compounds like these are usually named by simple benzene type naming (chlorobenzene and bromobenzene), the phenyl group naming is usually applied to benzene rings where a substituent with vi or more than carbons is fastened, such as in the diagram below.

Figure 21. Diagram of 2-phenyloctane.
Although the diagram higher up might be a picayune daunting to understand at first, it is non as difficult equally information technology seems afterwards careful assay of the structure is fabricated. By looking for the longest concatenation in the chemical compound, it should be clear that the longest chain is eight (viii) carbons long (octane, as shown in light-green) and that a benzene ring is fastened to the second position of this longest chain (labeled in red). Equally this rule suggests that the benzene ring will act every bit a part grouping (a substituent) whenever a substituent of more half-dozen (half dozen) carbons is fastened to it, the proper name "benzene" is changed to phenyl and is used the aforementioned fashion as any other substituents, such as methyl , ethyl, or bromo. Putting it all together, the name can be derived equally: two-phenyloctane (phenyl is fastened at the 2nd position of the longest carbon chain, octane).
The Benzyl Group
The benzyl group (abbv. Bn), like to the phenyl group, is formed past manipulating the benzene ring. In the case of the benzyl group, it is formed by taking the phenyl group and calculation a CH2 group to where the hydrogen was removed. Its molecular fragment can be written as CviHfiveCH2-R, PhCH2-R, or Bn-R. Classification of benzyl group based compounds are very similar to the phenyl group compounds. For instance, a chlorine fastened to a benzyl group would simply be chosen benzyl chloride, whereas an OH group fastened to a benzyl group would simply be called benzyl booze.
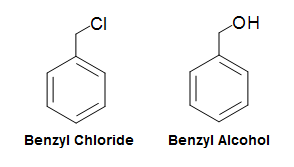
Figure 22. Benzyl Group Nomenclature
Additionally, other substituents tin can attach on the benzene ring in the presence of the benzyl grouping. An example of this tin be seen in the Figure below:
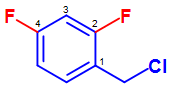
Figure 23. Classification of 2,4-difluorobenzyl chloride. Like to the base name nomenclatures system, the carbon in which th base of operations substitutent is fastened on the benzene ring is given the first priority and the rest of the substituents are given the everyman number social club possible.
Similar to the base proper name classification system, the carbon in which the base substituent is attached on the benzene ring is given the first priority and the rest of the substituents are given the everyman number order possible. Under this consideration, the above compound can be named: 2,iv-difluorobenzyl chloride.
Commonly Named Benzene Compounds Nomenclature Summary Flowchart
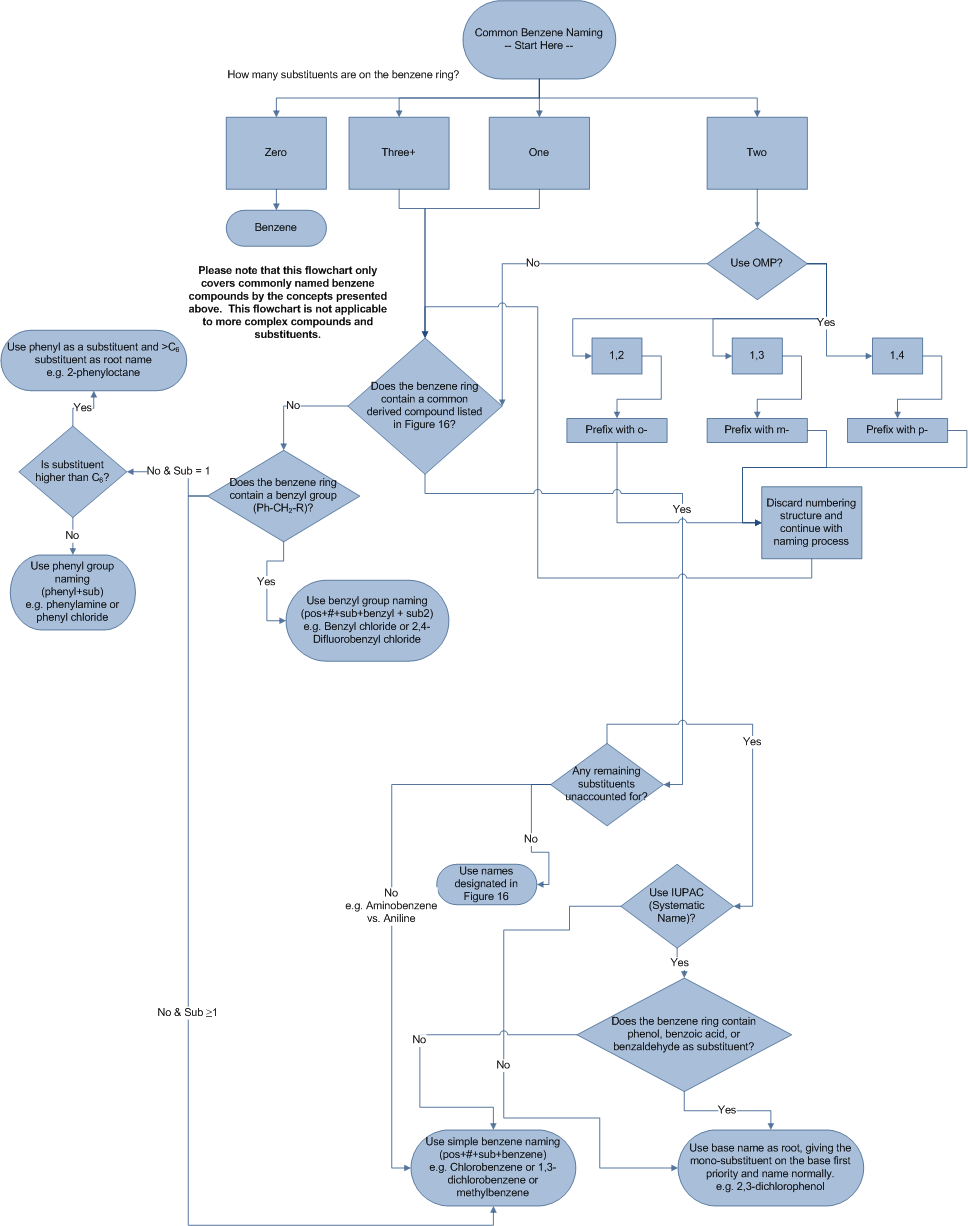
Summary Flowchart (Figure 24). Summary of nomenclature rules used in commonly benzene derived compounds.
Equally benzene derived compounds can be extremely complex, only compounds covered in this article and other commonly named compounds can be named using this flowchart.
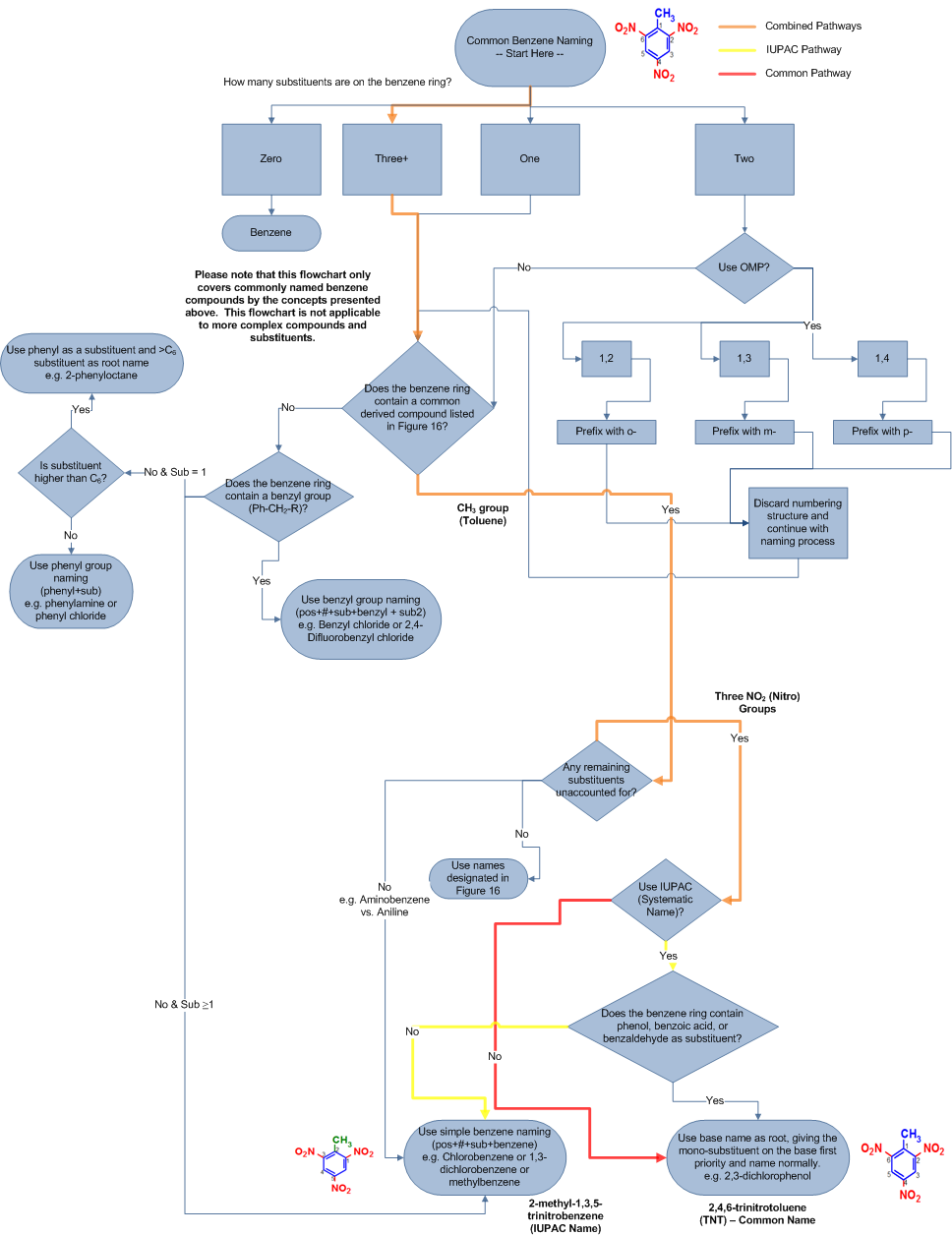
Conclusion of Mutual and Systematic Names using Flowchart
To demonstrate how this flowchart tin be used to proper name TNT in its common and systematic (IUPAC) name, a replica of the flowchart with the appropriate flow paths are shown below:
References
- Nicolaou, K. C., & Montagnon, T. (2008). Molecules That Changed the Globe. KGaA, Weinheim: Wiley-VCH. p. 54
- Pitman, V. (2004). Aromatherapy. Great britain, Britain: Nelson Thornes. p.135-136
- Burton, G. (2000). Chemic Ideas. Bicester, Oxon: Heinemann. p.290-292
- Vollhardt, K. P.C. & Shore, N. (2007). Organic Chemistry (vth Ed.). New York: W. H. Freeman. p. 667-669
- Schnaubelt, Grand. (1999). Medical Aromatherapy. Berkeley, CA: Frog Books. p. 211-213
- Patrick, Yard. L. (2004). Organic Chemistry. New York, NY: Taylor & Francis. p. 135-136
- Talbott, S. M. (2002). A Guide to Understanding Dietary Supplements. Binghamton, NY: Haworth Printing. p. 616-619
- Lifton, R. J. (2000). The Nazi doctors. New York, NY: Basic Books. p. 255-261
- Myers, R. L., & Myers, R. L. (2007). The 100 almost important chemical compounds. Westport, CT: Greenwood Publishing Group. p. 281-282
Do Problems
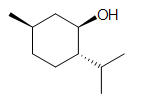
Q1) (Truthful/Faux) The compound above contains a benzene band and thus is aromatic.
Q2) Benzene unusual stability is caused by how many conjugated pi bonds in its cyclic ring? ____
Q3) Menthol, a topical analgesic used in many ointments for the relief of pain, releases a peppermint aroma upon exposure to the air. Based on this conclusion, can yous imply that a benzene band is present in its chemical structure? Why or why not?
Q4) 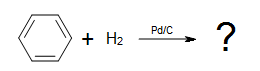
Q5) At normal conditions, benzene has ___ resonance structures.
Q6) Which of the following name(south) is/are correct for the following compound?
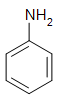
a) nitrohydride benzene
b) phenylamine
c) phenylamide
d) aniline
e) nitrogenhydrogen benzene
f) All of the above is correct
Q7) Catechumen 1,4-dimethylbenzene into its common name.
Q8) TNT'due south mutual proper name is: ______________________________
Q9) Proper name the following compound using OMP nomenclature:
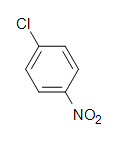
Q10) Depict the construction of 2,4-dinitrotoluene.
Q11) Proper noun the following compound:
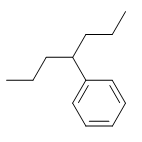
Q12) Which of the following is the correct name for the post-obit compound?

a) 3,iv-difluorobenzyl bromide
b) 1,2-difluorobenzyl bromide
c) iv,five-difluorobenzyl bromide
d) i,2-difluoroethyl bromide
e) 5,half dozen-difluoroethyl bromide
f) 4,v-difluoroethyl bromide
Q13) (True/Imitation) Benzyl chloride tin can be abbreviated Bz-Cl.
Q14) Benzoic Acrid has what R group attached to its phenyl functional group?
Q15) (True/Simulated) A single aromatic chemical compound can accept multiple names indicating its structure.
Q16) List the respective positions for the OMP arrangement (o-, yard-, p-).
Q17) A scientist has conducted an experiment on an unknown compound. He was able to determine that the unknown compound contains a cyclic band in its structure as well as an alcohol (-OH) grouping attached to the ring. What is the unknown compound?
a) Cyclohexanol
b) Cyclicheptanol
c) Phenol
d) Methanol
e) Bleach
f) Cannot determine from the above information
Q18) Which of the post-obit statements is imitation for the compound, phenol?
a) Phenol is a benzene derived compound.
b) Phenol can be fabricated past attaching an -OH grouping to a phenyl group.
c) Phenol is highly toxic to the body even in small doses.
d) Phenol can be used as a catalyst in the hydrogenation of benzene into cyclohexane.
e) Phenol is used equally an antiseptic in minute doses.
f) Phenol is amidst 1 of the 3 mutual names retained in the IUPAC nomenclature.
Reply Central to Practice Questions
Q1) Imitation, this compound does not contain a benzene band in its structure.
Q2) 3
Q3) No, a substance that is fragrant does not imply a benzene ring is in its structure. See camphor instance (Effigy one)
Q4) No reaction, benzene requires a special goad to be hydrogenated due to its unusual stability given by its three conjugated pi bonds.
Q5) 2
Q6) b, d
Q7) p-Xylene
Q8) ii,4,6-trinitrotoluene
Q9) p-chloronitrobenzene
Q10) 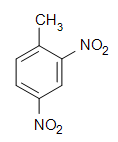
Q11) four-phenylheptane
Q12) a
Q13) Imitation, the correct abridgement for the benzyl group is Bn, not Bz. The correct abbreviation for Benzyl chloride is Bn-Cl.
Q14) COOH
Q15) True. TNT, for example, has the common name 2,4,6-trinitrotoluene and its systematic name is 2-methyl-i,3,5-trinitrobenzene.
Q16) Ortho - 1,2 ; Meta - 1,iii ; Para - one,four
Q17) The correct respond is f). We cannot make up one's mind what structure this is since the question does non tell usa what kind of cyclic ring the -OH grouping is attached on. Just every bit cyclohexane can be circadian, benzene and cycloheptane tin can too exist circadian.
Q18) d
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Arenes/Nomenclature_of_Arenes
0 Response to "Select the Name of the Family to Which the Following Compound Belongs"
Post a Comment